
Our Solutions

How We Make an Impact
We work to remove barriers to quality education and level the playing field for kids in need. At the heart of our work are the 600,000 members of the First Book Network, the largest online community of individual educators, professionals and volunteers dedicated to supporting children in need across North America. This network is the key to creating lasting change. We conduct research studies that aggregate their voices to identify barriers to education and inform strategic solutions. To address their needs, we provide free and low-cost books and resources through the First Book Marketplace, and access to leading experts and best-practice solutions through the First Book Accelerator.
Explore more: Our Projects | Featured Stories | Our Model of Change
Our Work

Reading Universe
An innovative, digital platform created to support educators by providing evidence-based guidance and tools for teaching students how to read and write.

The Impact of Book Bans on Educators and Students
First Book Research & Insights study reveals how educators and their students are responding to book bans.

Diverse Books Impact on Student Reading
First Book Research & Insights study reveals the impact increasing access to diverse books in classroom environments has on student reading and students’ reading scores.

Grants Deliver on Books
Grants promoting quality education, distribute 75,000 new books to increase kids’ excitement for reading.
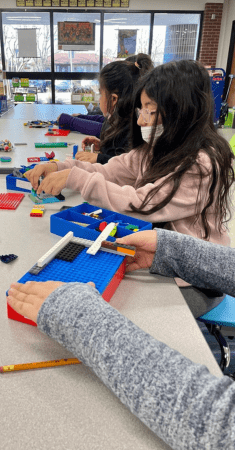
Learning Through Play Resources
Resources created to support educators in teaching students about environmental and societal challenges and encouraging them to explore solutions.

Building Literacy Rich Classrooms
Research & Insights study and Accelerator resource to help educators evaluate the quality of their classroom libraries.

Student Mental Health
Research & Insights study and Accelerator resource to address student mental wellness in classrooms.

Time for Change
An initiative designed to further changemaking, including resources for educators that suggests practical ways to inspire students to become changemakers for their schools and communities.

Magic of Storytelling
A national campaign to help distribute more than 80 million books to children in need, across the U.S. In partnership with Disney.

Stories for All Project™
Ensuring children have access to books where they can see themselves, their neighbors and the world around them, made available and affordable to educators supporting kids in need.

Grief, Loss & Healing
Equipping educators with resources to support children who are experiencing grief and loss. In partnership with New York Life Foundation.
